
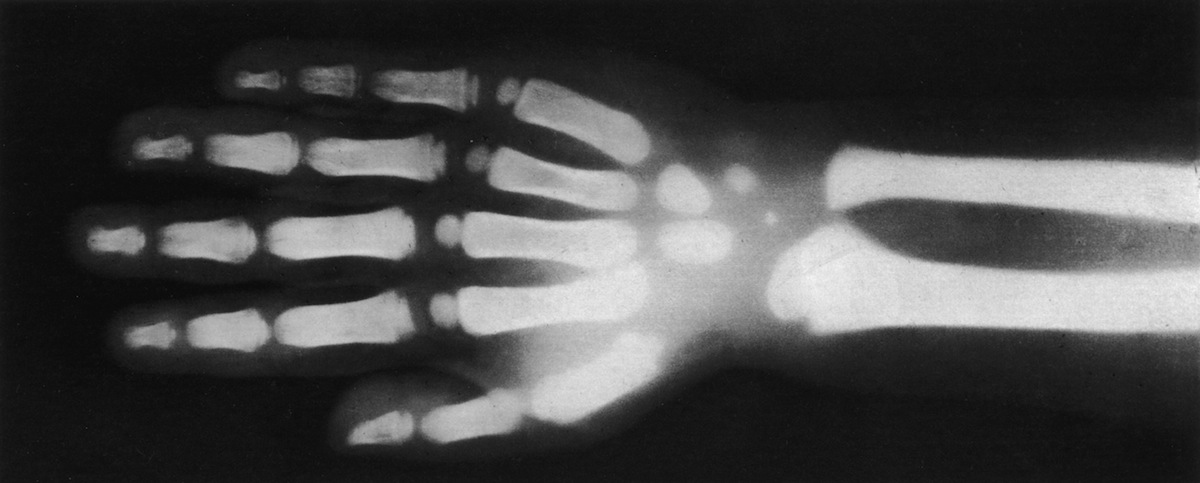
A small amount of energy is transformed into X-rays.

When the electrons hit the far end of the tube they give up all the energy they carry due to their speed and this is changed to other forms such as heat. In a cathode ray tube, electrons are accelerated from one end of the tube to the other using an electric field. How are electrons accelerated in a cathode ray tube? Who was the inventor of the cathode ray tube?Ĭathode Ray Tube The cathode ray tube (CRT), invented in 1897 by the German physicist Karl Ferdinand Braun, is an evacuated glass envelope containing an electron gun a source of electrons and a fluorescent light, usually with internal or external means to accelerate and redirect the electrons. Cathode ray tubes are sealed glass tubes from which most of the air has been evacuated. Thomson began experimenting with cathode ray tubes. Thomson and the discovery of the electron In the late century, physicist J.J. Who was the first scientist to discover the electron? He was even awarded a Nobel Prize in physics for this discovery and his work on the conduction During his experiment he discovered electron and it is one of the most important discoveries in the history of physics. Thomson experimenting with cathode ray tubes. Why was the cathode ray experiment so important?Ĭathode ray experiment was a result of English physicists named J. These rays are electrons that are actually produced from the gas ionization inside the tube. The electrons that were ejected from gas ionization travel to the anode. So those rays strike and ionize the gas sample inside the container. Thomson used the cathode ray tube to determine that atoms had small negatively charged particles inside of them, which he called “electrons.” Why are cathode rays produced?Ĭathode rays come from the cathode, because the cathode is charged negatively. Electrons were first discovered as the constituents of cathode rays. What did the cathode ray experiment discover?Ĭathode rays carry electronic currents through the tube. The nucleus of an atom is a combination of roughly equal numbers of protons and neutrons held together by the strong nuclear force. Protons combine with electrons and (usually) neutrons to make atoms. Why are protons neutrons and electrons important?Ī proton is one of the most important types of subatomic particles. Protons and neutrons are found in the nucleus of an atom. How did cathode ray tube experiment lead to the conclusion that atoms contain electrons? because Thomson saw the ray move from the cathode to the anode so the particles have negatively charge. How did the cathode ray tube experiment lead to the conclusion that atoms contain electrons? Thomson also concluded that electrons were part of the atom.

In 1897 he showed,through experiments with cathode rays, particles called electrons carry electricity. He developed the idea that electricity was transmitted by a charged smallest unit. Research with cathode rays led him to the discovery of electrons. How did his work with cathode ray tubes lead to his groundbreaking discovery? Thomson in Cathode Ray Tube (CRT) experiment. What experiment led to the discovery of the electron?Įlectron was discovered by J. The discovery of the electron, and the application of the electron ideas, first to gas discharges, then to radioactivity, spectroscopy and atomic structure, opened a most rapid advance in physical science, leading up to contemporary views of atomic structure and of chemistry. Why was the discovery of the electron important? Their work culminated in the discovery by English physicist J.J. Thomson proposed the plum pudding model of the atom, which had negatively-charged electrons embedded within a positively-charged “soup.” What scientist performed the cathode ray experiment leading to the discovery of electrons?ĭuring the 1880s and ’90s scientists searched cathode rays for the carrier of the electrical properties in matter. Thomson’s experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons. Why is the cathode ray experiment important?


 0 kommentar(er)
0 kommentar(er)
